The doctors and nurses didn’t believe Tomisa Starr was having trouble breathing.
Two years ago, Starr, 61, of Sacramento, California, was in the hospital for a spike in her blood pressure. She has multiple chronic health problems, including heart failure, and uses an oxygen tank at home.
But her request for supplemental oxygen while hospitalized was denied, Starr said, because readings from a pulse oximeter on her finger falsely indicated that she was getting plenty of air on her own.
Starr, who is Black, said she told the care team about research showing that the devices, which measure oxygen levels in the blood, may not work as well on people with darker skin and potentially make those patients seem healthier than they really are.
Their response, Starr said, was shocking.
“The provider demanded to know, ‘Who told you that?’” she said. “Here I am in the hospital, and I have to defend myself, like I’m in court.”

The skepticism Starr said she faced from doctors about potentially false pulse oximeter readings in Black Americans is not unique.
“I give talks on this all the time to physicians who should know the research, and they’re always blown away,” said Dr. Hugh Cassiere, a critical care physician at South Shore University Hospital in New York. “This device has built-in racial disparities that have been ignored for years.” Cassiere also heads a committee of independent experts assembled by the FDA to look into the pulse oximetry issue.
Potential problems with the measurement devices have been known for decades. The FDA began to develop guidance for manufacturers to address the issues in 2013, but little has been done since.
It wasn’t until the pandemic that the real dangers to Black patients became clear. In 2021, the Food and Drug Administration warned about flawed pulse oximeter readings on darker skin.
Last week, the FDA issued draft guidance for manufacturers to drastically expand the skin tone varieties when testing their devices and show they work equally for all skin pigments.
Historically, companies haven’t been required to include a significant number of dark-skinned people in studies of pulse oximeters.
“Quite frankly, how we’ve measured it hasn’t been consistent or done in a very valid or systematic way,” the head of the FDA’s Center for Devices and Radiological Health, Dr. Michelle Tarver, told NBC News in an interview before the draft guidance was issued.
Cassiere expressed support for the new recommendations. “This is light years ahead of the 2013 guidance,” he said.
The Food and Drug Administration doesn’t approve or authorize all pulse oximeters on the market. Devices marketed for “general wellness” among hikers, cyclists or other athletes who might want to estimate their blood oxygenation, for example, aren’t regulated and should never be used to diagnose or monitor health problems, the FDA said.
Pulse oximeters specifically intended for medical use do fall under FDA purview. It’s these device manufacturers that the agency is targeting with its draft guidance.
How pulse oximeters work
The gold standard and most accurate way of testing whether a person has normal oxygen levels is to stick a needle into a person’s wrist and draw blood.
An arterial blood draw, as it’s called, is invasive and painful. But it also gives doctors an essential clue as to how well a patient’s lungs are functioning, providing insight into which patients should be hospitalized and receive supplemental oxygen or other treatment.
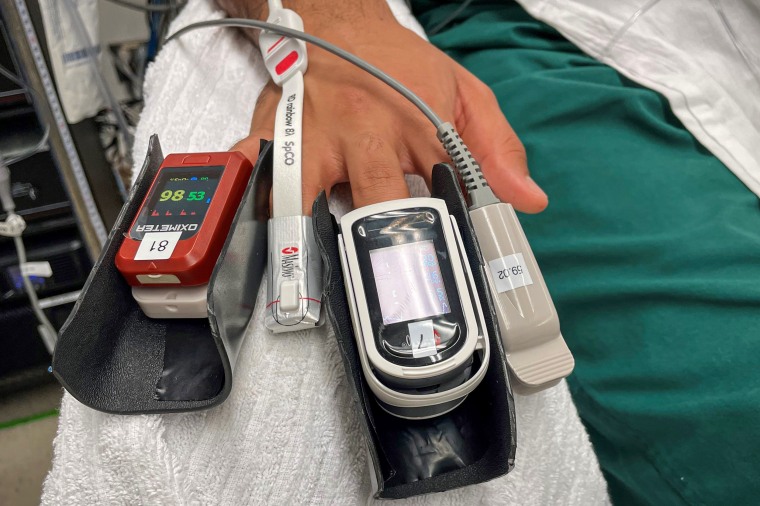



Pulse oximeters came along in the 1980s as an easy and painless alternative. The device shines a light through the fingertip, seeking out oxygen-rich blood. The more light that’s absorbed, the better, in theory.
But that light is also absorbed by melanin, or pigment, in the skin. As a result, Black and brown people are more likely to get pulse oximeter readings that show their blood oxygen saturation is higher than it really is. That is, they could be struggling for air despite normal pulse oximeter results.
The potential for racial disparities in pulse oximetry was first revealed in a study published 34 years ago. “That should have gotten the FDA’s attention,” Cassiere said.
It was largely disregarded.
“I went to medical school at Howard University, which is a historically Black college, and this never, ever came up — never in training, never in practice,” said Dr. Noha Aboelata, founder and chief operating officer of Roots Community Health in Oakland, California. “I never knew anything about it until the first year of the pandemic.”
The light bulb moment
It was Dr. Thomas Valley and his colleagues at the University of Michigan who reignited rumblings about the discrepancy in 2020 as they were inundated with Covid patients. The vast majority of the sickest patients were Black.
“We started to notice that the numbers on the screen for pulse oximeters weren’t matching what we were seeing in arterial blood,” Valley said. “We would go from room to room to room and see that these numbers didn’t look quite right.”
The team figured it was an anomaly caused by Covid — a brand-new virus the world hadn’t seen before.
“It wasn’t until several months later that a light bulb went off,” Valley said. “This isn’t a Covid problem. This is a ‘color of one’s skin’ problem.”
His team published its findings about racial biases in pulse oximeter readings in December 2020. It was this study, published in the prestigious New England Journal of Medicine, that Starr used to alert her doctors.
While there’s no direct link between faulty pulse oximeter readings and Covid deaths, additional research has found that people of color were more likely to die of Covid than white people.
The apparent disparities go beyond treatment for Covid.
The faulty pulse oximeter readings for patients of color “could preclude Black patients from being candidates for advanced therapy” such as heart pumps or heart transplants, said Sarah Adie, associate director of innovation at the University of Michigan Health Frankel Cardiovascular Center. She is a co-author of a study published in 2024 that found unreliable pulse oximeter readings might limit the way Black patients with heart failure qualify for those potentially lifesaving procedures.
Valley published additional research finding that a majority of pulse oximeter studies showed a bias against people with darker skin tones.
Is skin pigmentation the ultimate problem with pulse oximeters? Maybe, maybe not.
“The question that everybody wants to know right now is: Which devices work equally well, regardless of skin pigment?” said Dr. Michael Lipnick, an anesthesiologist at the University of California, San Francisco. His team is analyzing results from more than 50 pulse oximeters in an attempt to answer that question.
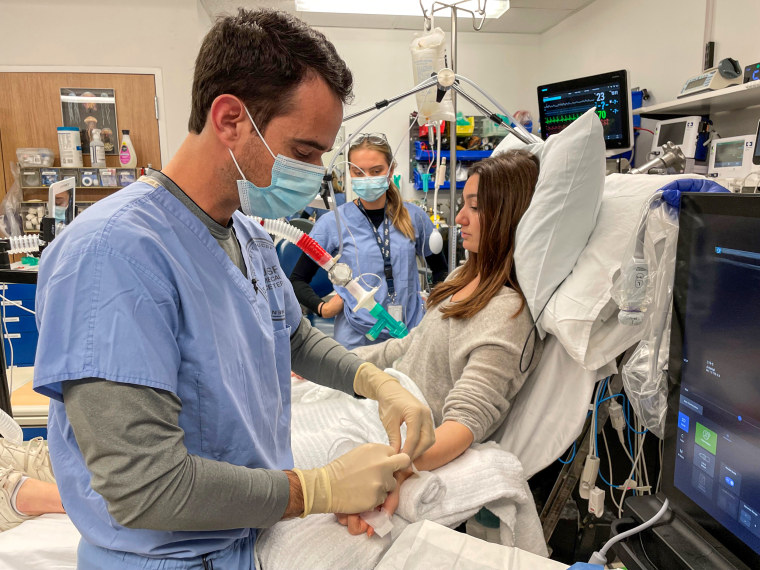



Under careful watch, UCSF researchers attach a variety of pulse oximeters to study participants’ fingers, then lower their oxygen levels to a point where the devices should signal a problem.
They expected that some devices — cheaper devices, perhaps — would perform poorly overall.
That’s not necessarily the case. “Some devices perform equally well regardless of skin pigment, whereas others don’t,” Lipnick said. “We don’t understand why.”
Measures of how well a person is circulating oxygen-rich blood through the body, called perfusion, could also play a role. That’s not a condition specific to skin color. It could very well be years before the team understands the differences in pulse oximeter readings and why some perform poorly on people of color.
Delays in answers — or action — do not sit well with doctors who treat mostly Black patients.
“We thought that there would be some kind of drastic action,” said Aboelata of Oakland’s Roots Community Health. “Given how diverse of a country we have, how could we possibly have a medical device that only works well on white skin?”
The Roots clinic has sued 13 companies that make or sell pulse oximeters.
So far, Medtronic has agreed to warn California hospitals that its devices may give higher readings for patients with darker skin. Four other companies, Veridian Healthcare, Compass Health Brands, Gurin Products and Zewa, have agreed to add warning labels for consumers in California.
But patients like Starr still rely on pulse oximeters, knowing they could provide faulty results. She has no other option, she said. “They’re all I have.”